Electrocatalyst ANL on:
[Wikipedia]
[Google]
[Amazon]
An electrocatalyst is a
 Electrocatalysts can be evaluated according to three figures of merit: activity, stability, and selectivity. The activity of electrocatalysts can be assessed quantitatively by understanding how much current density is generated, and therefore how fast a reaction is taking place, for a given applied potential. This relationship is described with the
Electrocatalysts can be evaluated according to three figures of merit: activity, stability, and selectivity. The activity of electrocatalysts can be assessed quantitatively by understanding how much current density is generated, and therefore how fast a reaction is taking place, for a given applied potential. This relationship is described with the 

 Also, higher reaction rates can be achieved by precisely controlling the arrangement of surface atoms: indeed, in nanometric systems, the number of available reaction sites is a better parameter than the exposed surface area in order to estimate electrocatalytic activity. Sites are the positions where the reaction could take place; the likelihood of a reaction to occur in a certain site depends on the electronic structure of the catalyst, which determines the
Also, higher reaction rates can be achieved by precisely controlling the arrangement of surface atoms: indeed, in nanometric systems, the number of available reaction sites is a better parameter than the exposed surface area in order to estimate electrocatalytic activity. Sites are the positions where the reaction could take place; the likelihood of a reaction to occur in a certain site depends on the electronic structure of the catalyst, which determines the
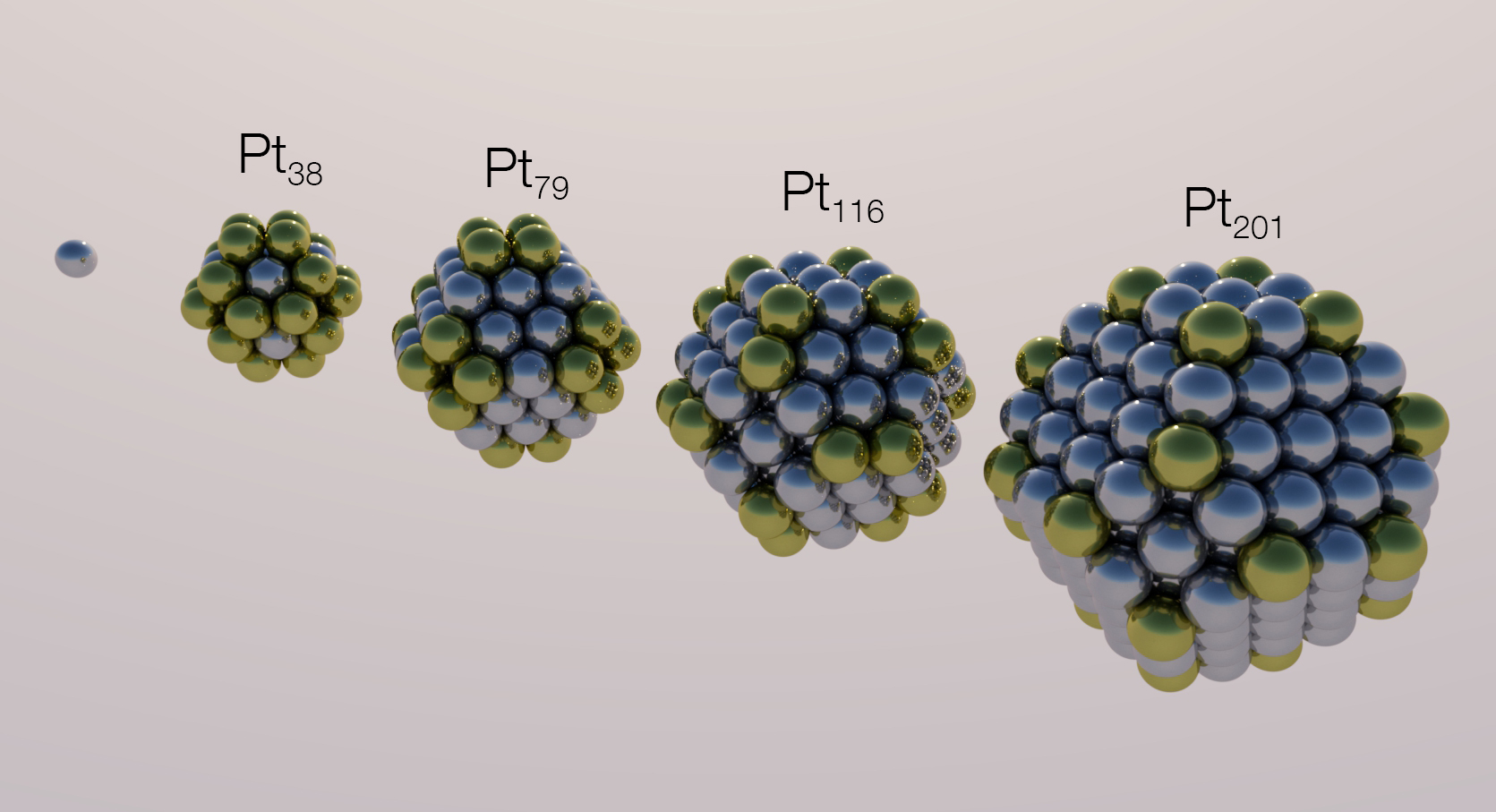 The interest in reducing as much as possible the costs of the catalyst for electrochemical processes led to the use of fine catalyst powders since the
The interest in reducing as much as possible the costs of the catalyst for electrochemical processes led to the use of fine catalyst powders since the
 Hydrogen and oxygen can be combined through by the use of a fuel cell. In this process, the reaction is broken into two half reactions which occur at separate electrodes. In this situation the reactant's energy is directly converted to electricity. Useful energy can be obtained from the thermal heat of this reaction through an
Hydrogen and oxygen can be combined through by the use of a fuel cell. In this process, the reaction is broken into two half reactions which occur at separate electrodes. In this situation the reactant's energy is directly converted to electricity. Useful energy can be obtained from the thermal heat of this reaction through an
catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials d ...
surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst can be heterogeneous such as a platinized electrode. Homogeneous electrocatalysts, which are soluble, assist in transferring electrons between the electrode and reactants, and/or facilitate an intermediate chemical transformation described by an overall half reaction
A half reaction (or half-cell reaction) is either the oxidation or reduction reaction component of a redox reaction. A half reaction is obtained by considering the change in oxidation states of individual substances involved in the redox reaction. ...
. Major challenges in electrocatalysts focus on fuel cells.
Practical electrocatalysts
Chloralkali process
Thechloralkali process
The chloralkali process (also chlor-alkali and chlor alkali) is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide (caustic soda), which are commodit ...
is a large scale application that uses electrocatalysts. This technology supplies most of the chlorine and sodium hydroxide required by many industries. The cathode is a mixed metal oxide clad titanium anode (also called a dimensionally stable anode).
Electrofluorination
Many organofluorine compounds are produced by electrofluorination. One manifestation of this technology is the Simons process, which can be described as: :R3C–H + HF → R3C–F + H2 In the course of a typical synthesis, this reaction occurs once for each C–H bond in the precursor. The cell potential is maintained near 5–6 V. Theanode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic ...
, the electrocatalyst, is nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
-plated.
Hydrodimerization of acrylonitrile
Acrylonitrile is converted toadiponitrile
Adiponitrile is an organic compound with the chemical formula (CH2)4(CN)2. This viscous, colourless dinitrile is an important precursor to the polymer nylon 66. In 2005, about one million tonnes of adiponitrile were produced.M. T. Musser, "Adip ...
on an industrial scale via electrocatalysis.
Background and theory
In general, acatalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
is an agent that increases the speed of a chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
without being consumed by a reaction. Thermodynamically, a catalyst lowers the activation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules p ...
required for a chemical reaction to take place. An electrocatalyst is a catalyst that affects the activation energy of an electrochemical reaction. Shown below is the activation energy of chemical reactions as it relates to the energies of products and reactants. The activation energy in electrochemical processes is related to the potential
Potential generally refers to a currently unrealized ability. The term is used in a wide variety of fields, from physics to the social sciences to indicate things that are in a state where they are able to change in ways ranging from the simple r ...
, i.e. voltage, at which a reaction occurs. Thus, electrocatalysts frequently change the potential at which oxidation and reduction processes are observed. Alternatively, an electrocatalyst can be thought of as an agent that facilitates a specific chemical interaction at an electrode surface. Given that electrochemical reactions occur when electrons are passed from one chemical species to another, favorable interactions at an electrode surface increase the likelihood of electrochemical transformations occurring, thus reducing the potential required to achieve these transformations.
 Electrocatalysts can be evaluated according to three figures of merit: activity, stability, and selectivity. The activity of electrocatalysts can be assessed quantitatively by understanding how much current density is generated, and therefore how fast a reaction is taking place, for a given applied potential. This relationship is described with the
Electrocatalysts can be evaluated according to three figures of merit: activity, stability, and selectivity. The activity of electrocatalysts can be assessed quantitatively by understanding how much current density is generated, and therefore how fast a reaction is taking place, for a given applied potential. This relationship is described with the Tafel equation
The Tafel equation is an equation in electrochemical kinetics relating the rate of an electrochemical reaction to the overpotential. The Tafel equation was first deduced experimentally and was later shown to have a theoretical justification. The ...
. In assessing the stability of electrocatalysts, the ability of catalysts to withstand the potentials at which transformations are occurring is crucial. The selectivity of electrocatalysts refers to their preferential interaction with particular substrates, and their generation of a single product. Selectivity can be quantitatively assessed through a selectivity coefficient, which compares the response of the material to the desired analyte or substrate with the response to other interferents.
In many electrochemical systems, including galvanic cell
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous Oxidation-Reduction reactions. A common apparatus ...
s, fuel cells and various forms of electrolytic cell
An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy to force a chemical reaction that would not otherwise occur. The external energy source is a voltage applied between the cell′s two electrod ...
s, a drawback is that they can suffer from high activation barriers. The energy diverted to overcome these activation barriers is transformed into heat. In most exothermic combustion reactions this heat would simply propagate the reaction catalytically. In a redox reaction, this heat is a useless byproduct lost to the system. The extra energy required to overcome kinetic barriers is usually described in terms of low faradaic efficiency and high overpotential
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly rela ...
s. In these systems, each of the two electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials d ...
s and its associated half-cell
In electrochemistry, a half-cell is a structure that contains a conductive electrode and a surrounding conductive electrolyte separated by a naturally occurring Helmholtz double layer. Chemical reactions within this layer momentarily pump electri ...
would require its own specialized electrocatalyst.
Half-reactions involving multiple steps, multiple electron transfers, and the evolution or consumption of gases in their overall chemical transformations, will often have considerable kinetic barriers. Furthermore, there is often more than one possible reaction at the surface of an electrode. For example, during the electrolysis of water
Electrolysis of water, also known as electrochemical water splitting, is the process of using electricity to decompose water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, or remi ...
, the anode can oxidize water through a two electron process to hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%â ...
or a four electron process to oxygen. The presence of an electrocatalyst could facilitate either of the reaction pathways.

Homogeneous electrocatalysts
A homogeneous electrocatalyst is one that is present in the same phase of matter as the reactants, for example, a water-soluble coordination complex catalyzing an electrochemical conversion in solution. This technology is not practiced commercially, but is of research interest.Synthetic coordination complexes
Manycoordination complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as '' ligands'' or complexing agents. ...
es catalyze electrochemical reactions, but only heterogeneous catalysts are of commercial value.

Enzymes
Someenzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s can function as electrocatalysts. Nitrogenase
Nitrogenases are enzymes () that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria) and rhizobacteria. These enzymes are responsible for the reduction of nitrogen (N2) to ammonia (NH3). Nitrogenases are the only fa ...
, an enzyme that contains a MoFe cluster, can be leveraged to fix atmospheric nitrogen, i.e. convert nitrogen gas into molecules such as ammonia. Immobilizing the protein onto an electrode surface and employing an electron mediator greatly improves the efficiency of this process. The effectiveness of bioelectrocatalysts generally depends on the ease of electron transport between the active site of the enzyme and the electrode surface. Other enzymes provide insight for the development of synthetic catalysts. For example, formate dehydrogenase
Formate dehydrogenases are a set of enzymes that catalyse the oxidation of formate to carbon dioxide, donating the electrons to a second substrate, such as NAD+ in formate:NAD+ oxidoreductase () or to a cytochrome in formate:ferricytochrome-b1 o ...
, a nickel-containing enzyme, has inspired the development of synthetic complexes with similar molecular structures for use in CO2 reduction. Microbial fuel cell Microbial fuel cell (MFC) is a type of bioelectrochemical fuel cell system that
generates electric current by diverting electrons produced from the microbial oxidation of reduced compounds (also known as fuel or electron donor) on the anode to oxid ...
s are another way that biological systems can be leveraged for electrocatalytic applications. Microbial-based systems leverage the metabolic pathways of an entire organism, rather than the activity of a specific enzyme, meaning that they can catalyze a broad range of chemical reactions. Microbial fuel cells can derive current from the oxidation of substrates such as glucose, and be leveraged for processes such as CO2 reduction.
Heterogeneous electrocatalysts
A heterogeneous electrocatalyst is one that is present in a different phase of matter from the reactants, for example, a solid surface catalyzing a reaction in solution. Different types of heterogeneous electrocatalyst materials are shown above in green. Since heterogeneous electrocatalytic reactions need an electron transfer between the solid catalyst (typically a metal) and the electrolyte, which can be a liquid solution but also a polymer or a ceramic capable of ionic conduction, the reaction kinetics depend on both the catalyst and the electrolyte as well as on theinterface
Interface or interfacing may refer to:
Academic journals
* ''Interface'' (journal), by the Electrochemical Society
* '' Interface, Journal of Applied Linguistics'', now merged with ''ITL International Journal of Applied Linguistics''
* '' Int ...
between them. The nature of the electrocatalyst surface determines some properties of the reaction including rate and selectivity.
Bulk materials
Electrocatalysis can occur at the surface of some bulk materials, such as platinum metal. Bulk metal surfaces of gold have been employed for the decomposition methanol for hydrogen production. Water electrolysis is conventionally conducted at inert bulk metal electrodes such as platinum or iridium. The activity of an electrocatalyst can be tuned with a chemical modification, commonly obtained by alloying two or more metals. This is due to a change in the electronic structure, especially in the d band which is considered to be responsible for the catalytic properties of noble metals.Nanomaterials
Nanoparticles
A variety ofnanoparticle
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 10 ...
materials have been demonstrated to promote various electrochemical reactions, although none have been commercialized. These catalysts can be tuned with respect to their size and shape, as well as the surface strain. Also, higher reaction rates can be achieved by precisely controlling the arrangement of surface atoms: indeed, in nanometric systems, the number of available reaction sites is a better parameter than the exposed surface area in order to estimate electrocatalytic activity. Sites are the positions where the reaction could take place; the likelihood of a reaction to occur in a certain site depends on the electronic structure of the catalyst, which determines the
Also, higher reaction rates can be achieved by precisely controlling the arrangement of surface atoms: indeed, in nanometric systems, the number of available reaction sites is a better parameter than the exposed surface area in order to estimate electrocatalytic activity. Sites are the positions where the reaction could take place; the likelihood of a reaction to occur in a certain site depends on the electronic structure of the catalyst, which determines the adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which ...
energy of the reactants together with many other variables not yet fully clarified.
According to the TSK model, the catalyst surface atoms can be classified as terrace, step or kink atoms according to their position, each characterized by a different coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central io ...
. In principle, atoms with lower coordination number (kinks and defects) tend to be more reactive and therefore adsorb the reactants more easily: this may promote kinetics but could also depress it if the adsorbing species isn't the reactant, thus inactivating the catalyst. Advances in nanotechnology make it possible to surface engineer the catalyst so that just some desired crystal planes are exposed to reactants, maximizing the number of effective reaction sites for the desired reaction.
To date, a generalized surface dependence mechanism cannot be formulated since every surface effect is strongly reaction-specific. A few classifications of reactions based on their surface dependence have been proposed but there are still many exceptions that do not fall into them.
= Particle size effect
= The interest in reducing as much as possible the costs of the catalyst for electrochemical processes led to the use of fine catalyst powders since the
The interest in reducing as much as possible the costs of the catalyst for electrochemical processes led to the use of fine catalyst powders since the specific surface area
Specific surface area (SSA) is a property of solids defined as the total surface area of a material per unit of mass, (with units of m2/kg or m2/g) or solid or bulk volume (units of m2/m3 or m−1).
It is a physical value that can be used to dete ...
increases as the average particle size decreases. For instance, most common PEM fuel cells and electrolyzers design is based on a polymeric membrane charged in platinum nanoparticles as an electrocatalyst (the so-called platinum black
Platinum black (Pt black) is a fine powder of platinum with good catalytic properties. The name of platinum black is due to its black color. It is used in many ways; as a thin film electrode, a fuel cell membrane catalyst, or as a catalytic igniti ...
).
Although the surface area to volume ratio
The surface-area-to-volume ratio, also called the surface-to-volume ratio and variously denoted sa/vol or SA:V, is the amount of surface area per unit volume of an object or collection of objects.
SA:V is an important concept in science and engi ...
is commonly considered to be the main parameter relating electrocatalyst size with its activity, to understand the particle-size effect, several more phenomena need to be taken into account:
* '' Equilibrium shape'': for any given size of a nanoparticle there is an equilibrium shape which exactly determines its crystal planes
* ''Reaction sites relative number'': a given size for a nanoparticle corresponds to a certain number of surface atoms and only some of them host a reaction site
* ''Electronic structure
In quantum chemistry, electronic structure is the state of motion of electrons in an electrostatic field created by stationary nuclei. The term encompasses both the wave functions of the electrons and the energies associated with them. Electr ...
'': below a certain size, the work function
In solid-state physics, the work function (sometimes spelt workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" ...
of a nanoparticle changes and its band structure fades away
* '' Defects'': the crystal lattice of a small nanoparticle is perfect; thus, reactions enhanced by defects as reaction sites get slowed down as the particle size decreases
* ''Stability'': small nanoparticles have the tendency to lose mass due to the diffusion of their atoms towards bigger particles, according to the Ostwald ripening
Ostwald ripening is a phenomenon observed in solid solutions or liquid sols that describes the change of an inhomogeneous structure over time, i.e., small crystals or sol particles dissolve, and redeposit onto larger crystals or sol particles ...
phenomenon
* ''Capping agents'': in order to stabilize nanoparticles it is necessary a capping layer, therefore part of their surface is unavailable for reactants
* '' Support'': nanoparticles are often fixed onto a support in order to stay in place, therefore part of their surface is unavailable for reactants
Carbon-based materials
Carbon nanotubes andgraphene
Graphene () is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure.
-based materials can be used as electrocatalysts. The carbon surfaces of graphene and carbon nanotubes are well suited to the adsorption of many chemical species, which can promote certain electrocatalytic reactions. In addition, their conductivity means they are good electrode materials. Carbon nanotubes have a very high surface area, maximizing surface sites at which electrochemical transformations can occur. Graphene can also serve as a platform for constructing composites with other kinds of nanomaterials such as single atom catalysts. Because of their conductivity, carbon-based materials can potentially replace metal electrodes to perform metal-free electrocatalysis.
Framework materials
Metal–organic framework, Metal—organic frameworks (MOFs), especially conductive frameworks, can be used as electrocatalysts for processes such as CO2 reduction andwater splitting
Water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen:
:2 H2O → 2 H2 + O2
Efficient and economical water splitting would be a technological breakthrough that could underpin a hydrogen economy, base ...
. MOFs provide potential active sites at both metal centers and organic ligand sites. They can also be functionalized, or encapsulate other materials such as nanoparticles. MOFs can also be combined with carbon-based materials to form electrocatalysts. Covalent organic frameworks (COFs), particularly those that contain metals, can also serve as electrocatalysts. COFs constructed from cobalt porphyrins demonstrated the ability to reduce carbon dioxide to carbon monoxide.
However, many MOFs are known unstable in chemical and electrochemical conditions, making it difficult to tell if MOFs are actually catalysts or precatalysts. The real active sites of MOFs during electrocatalysis need to be analyzed comprehensively.
Research on electrocatalysis
Water splitting / Hydrogen evolution
 Hydrogen and oxygen can be combined through by the use of a fuel cell. In this process, the reaction is broken into two half reactions which occur at separate electrodes. In this situation the reactant's energy is directly converted to electricity. Useful energy can be obtained from the thermal heat of this reaction through an
Hydrogen and oxygen can be combined through by the use of a fuel cell. In this process, the reaction is broken into two half reactions which occur at separate electrodes. In this situation the reactant's energy is directly converted to electricity. Useful energy can be obtained from the thermal heat of this reaction through an internal combustion engine
An internal combustion engine (ICE or IC engine) is a heat engine in which the combustion of a fuel occurs with an oxidizer (usually air) in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal c ...
with an upper efficiency of 60% (for compression ratio of 10 and specific heat ratio of 1.4) based on the Otto
Otto is a masculine German given name and a surname. It originates as an Old High German short form (variants ''Audo'', '' Odo'', ''Udo'') of Germanic names beginning in ''aud-'', an element meaning "wealth, prosperity".
The name is recorded f ...
thermodynamic cycle
A thermodynamic cycle consists of a linked sequence of thermodynamic processes that involve transfer of heat and work into and out of the system, while varying pressure, temperature, and other state variables within the system, and that eventual ...
. It is also possible to combine the hydrogen and oxygen through redox mechanism as in the case of a fuel cell. In this process, the reaction is broken into two half-reactions which occur at separate electrodes. In this situation the reactant's energy is directly converted to electricity.
The standard reduction potential of hydrogen is defined as 0V, and frequently referred to as the standard hydrogen electrode
The standard hydrogen electrode (abbreviated SHE), is a redox electrode which forms the basis of the thermodynamic scale of oxidation-reduction potentials. Its absolute electrode potential is estimated to be at 25 °C, but to form a basis ...
(SHE).
HER can be promoted by many catalysts.
Carbon dioxide reduction
Electrocatalysis for CO2 reduction is not practiced commercially but remains a topic of research. The reduction of CO2 into useable products is a potential way to combatclimate change
In common usage, climate change describes global warming—the ongoing increase in global average temperature—and its effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes to ...
. Electrocatalysts can promote the reduction of carbon dioxide into methanol and other useful fuel and stock chemicals. The most valuable reduction products of CO2 are those that have a higher energy content, meaning that they can be reused as fuels. Thus, catalyst development focuses on the production of products such as methane and methanol. Homogeneous catalysts, such as enzymes and synthetic coordination complexes have been employed for this purpose. A variety of nanomaterials have also been studied for CO2 reduction, including carbon-based materials and framework materials.
Ethanol-powered fuel cells
Aqueous solutions of methanol can decompose into CO2 hydrogen gas, and water. Although this process is thermodynamically favored, the activation barrier is extremely high, so in practice this reaction is not typically observed. However, electrocatalysts can speed up this reaction greatly, making methanol a possible route to hydrogen storage for fuel cells. Electrocatalysts such as gold, platinum, and various carbon-based materials have been shown to effectively catalyze this process. An electrocatalyst ofplatinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Pla ...
and rhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring i ...
on carbon backed tin-dioxide nanoparticles can break carbon bonds at room temperature with only carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
as a by-product, so that ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
can be oxidized into the necessary hydrogen ions and electrons required to create electricity.
Chemical synthesis
Electrocatalysts are used to promote certain chemical reactions to obtain synthetic products. Graphene and graphene oxides have shown promise as electrocatalytic materials for synthesis. Electrocatalytic methods also have potential for polymer synthesis. Electrocatalytic synthesis reactions can be performed under a constant current, constant potential, or constant cell-voltage conditions, depending on the scale and purpose of the reaction.Additional reading
*See also
*Electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
*Catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
*Electrolysis of water
Electrolysis of water, also known as electrochemical water splitting, is the process of using electricity to decompose water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, or remi ...
* Non-faradaic electrochemical modification of catalytic activity
*Tafel equation
The Tafel equation is an equation in electrochemical kinetics relating the rate of an electrochemical reaction to the overpotential. The Tafel equation was first deduced experimentally and was later shown to have a theoretical justification. The ...
References
{{Reflist Electrochemistry Catalysis